NAD+: What Science Says About the Hottest Longevity Molecule
8/8/20256 min read


Why Is NAD+ Everywhere?
If your body were a city, NAD+ would be its currency - traded in every district, from power plants (mitochondria) to repair crews (DNA repair enzymes) to the communication networks that keep it all running.
NAD+ is a coenzyme: it doesn’t do the work itself, but it enables thousands of reactions that keep us alive. It’s central to turning food into energy, repairing damaged DNA, and helping cells talk to each other. In longevity research, NAD+ is often called a “linchpin” - because so many age‑related changes link back to its steady decline over time.
That’s shortly the main reason, why with the rising interest in longevity, so does the one in NAD+.
What Exactly Is NAD+?
NAD+ stands for nicotinamide adenine dinucleotide, a molecule found in every living cell. It cycles between NAD+ (oxidized) and NADH (reduced) to shuttle electrons and keep metabolism moving. Beyond energy, NAD+ feeds the enzymes that repair DNA (PARPs), regulate stress and metabolism (sirtuins), and help maintain cellular balance.
Sirtuins are the direct "action" link between NAD+ and cellular health - they use NAD+ as fuel to perform their jobs (like managing gene expression and mitochondrial health). At the same time, the enzyme CD38 (a major NAD+ consumer) is activated by chronic, low-grade inflammation, a state often called "inflammaging”.
If ATP is your body’s “cash on hand,” NAD+ is the banking system that moves and allocates it where it’s needed most.
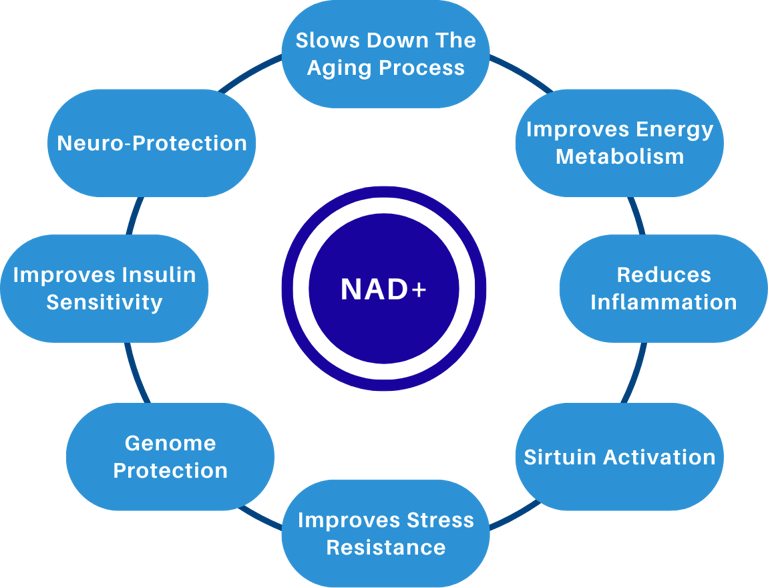
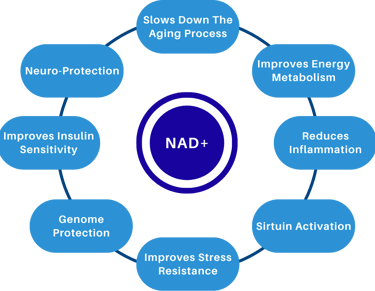
NAD+ and the Hallmarks of Aging
In our Hallmarks post, we saw how aging is a network of interconnected processes. NAD+ sits at the crossroads of several of them - mitochondrial function, DNA stability, protein maintenance, and cellular senescence are all influenced by NAD+ dependent pathways.
Why NAD+ Declines With Age?
From about midlife onward, NAD+ levels naturally drop - in some tissues, by as much as 50% or more. Several factors drive this decline:
Increased consumption - enzymes like PARPs and CD38 burn through NAD+ to repair DNA damage or manage inflammation. With age (and more cellular stress), these enzymes become overactive.
Reduced synthesis - the pathways that make NAD+ slow down.
Lifestyle stressors - poor sleep, chronic inflammation, high-sugar diets, and sedentary living further drain NAD+ stores.
It’s a double hit: supply lines slow while demand spikes. If we put it in more scientific way: The vast majority (over 85%) of our NAD+ is not made from food from scratch, but is recycled from nicotinamide (a byproduct of NAD+ consumption). This recycling process is called the salvage pathway, and its main rate-limiting enzyme is called NAMPT. A key reason NAD+ levels decline with age is that NAMPT levels and efficiency also decline.
By your 50s, NAD+ levels may be half of what they were in your 20s.
Can We Support or Boost NAD+?
Shortly - yes, to some level. How? Well, that’s a bit more complex question, but let’s run through it step by step:
“In animal and human studies, regular aerobic exercise has been shown to boost NAD+ levels by up to 30% in muscle tissue.” (Rajman et al., 2018; Cantó et al., 2015)
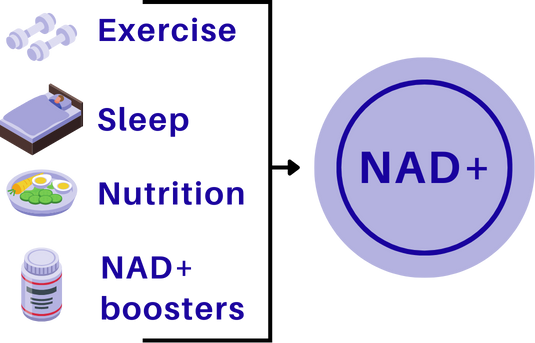
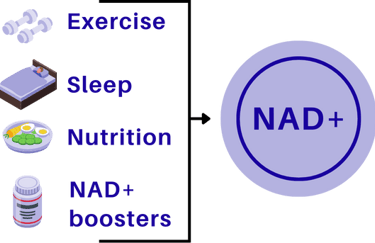
Lifestyle levers
Exercise - especially aerobic and interval training - stimulates NAD+ production and improves mitochondrial efficiency.
Caloric restriction / time-restricted eating - can reduce NAD+ consumption and promote repair over growth.
Circadian rhythm alignment - NAD+ metabolism follows daily cycles; regular sleep supports optimal levels.
Precursors - Building Blocks
We’ll explore NAD+ precursors like NR and NMN in depth later, but here’s the big picture:
Nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) are the two most studied direct precursors.
NR is known for its good oral stability and human safety data.
NMN also shows promise, but its absorption pathways are still debated.
Niacin and niacinamide - older, less expensive options, though higher doses can have side effects.
When the body processes NAD+ precursors like NR and NMN, the leftover nicotinamide needs to be neutralized and excreted. This process consumes methyl groups, which are vital for countless other bodily functions (like DNA regulation). Taking high doses of NAD+ precursors can potentially deplete these methyl groups. This is why many people who supplement with NMN or NR also take a "methyl donor" like trimethylglycine (TMG). We’ll compare mechanisms, clinical evidence, and practical considerations in separate deep‑dive posts.
Emerging Approaches
We’ll talk about it later in greater depth, but generally:
CD38 inhibitors - slowing one of the main NAD+ “consumers” in the body.
PARP modulation - balancing DNA repair demands without excessive NAD+ drain.
Gene therapy - experimental methods to enhance NAD+ biosynthesis directly in cells.
Think foundation first - sleep, movement, and nutrition - then consider targeted tools.
The Hype vs. The Science
NAD+ is essential for energy, repair, and cellular resilience - and its levels do decline with age. Early human work on precursors is promising but still evolving; long‑term outcomes remain to be proven. Treat NAD+ support as part of a broader healthy‑aging plan, not a standalone fix (Rajman et al., 2018).


Everyday Ways to Protect Your NAD+
Move daily, eat well, and keep a consistent sleep schedule - these support NAD+ naturally.
Consider NAD+ precursors only after lifestyle basics are solid.
If supplementing, choose quality-tested products and consult a healthcare provider.
We’ll dive deeper into NR and NMN in the coming weeks - including how to choose between them, dosing considerations, and what the best studies say so far.
The Road Ahead
Next up, we’ll dive into NR and NMN separately - how they work, what human studies show, how to think about dosing and quality, and who might benefit. We’ll also explore other strategies (like targeting CD38) and how to track whether your NAD+ support is doing anything meaningful for you.
The future of aging is here - and the choices you make today decide how you’ll meet it.
Important terms
ATP or adenosine triphosphate - a molecule that serves as the primary energy carrier in cells.
Linchpin - a person or thing vital to an enterprise or organization.
Sirtuins - a type of protein involved in regulating cellular processes including the ageing and death of cells and their resistance to stress.
Inflammaging - chronic, low-grade, systemic inflammation that occurs with aging, even in the absence of infection or overt injury.
Enzyme - An enzyme is a type of protein that dramatically speeds up chemical reactions inside a living organism. Essentially, it's a tiny helper that makes essential processes, like digesting food or building new cells, happen fast enough to support life.
Enzyme CD38 - an enzyme that increases as we age, destroying a vital molecule called NAD+ which is crucial for cellular energy and DNA repair. By depleting NAD+, high levels of CD38 accelerate the aging process and contribute to age-related health decline.


References
Camacho-Pereira, J., Tarragó, M. G., Chini, C. C. S., et al. (2016). CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through a SIRT3-Dependent Mechanism. Cell Metabolism, 23(6), 1127–1139.
Cantó, C., Menzies, K. J., & Auwerx, J. (2015). NAD+ metabolism and the control of energy homeostasis: A balancing act between mitochondria and the nucleus. Cell Metabolism, 22(1), 31–53.
Chini, E. N., Tarragó, M. G., & Chini, C. C. S. (2021). NAD and the aging process: Role in life, death and everything in between. Cell Metabolism, 33(5), 837–857.
Chu, X., & Raju, R. P. (2022). Regulation of NAD+ metabolism in aging and disease. Metabolism, 126, 154923.
Covarrubias, A. J., Perrone, R., Grozio, A., & Verdin, E. (2020). NAD+ metabolism and its roles in cellular processes during ageing. Nature Reviews Molecular Cell Biology, 22(2), 119–141.
Giner, M. P., Christen, S., Bartova, S., Makarov, M. V., Migaud, M. E., Cantó, C., & Moco, S. (2021). A method to monitor the NAD+ metabolome—from mechanistic to clinical applications. International Journal of Molecular Sciences, 22(19), 10598.
Imai, S., & Guarente, L. (2014). NAD+ and sirtuins in aging and disease. Trends in Cell Biology, 24(8), 464–471.
Mills, K. F., Yoshida, S., Stein, L. R., et al. (2016). Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metabolism, 24(6), 795–806.
Rajman, L., Chwalek, K., & Sinclair, D. A. (2018). Therapeutic potential of NAD‑boosting molecules: The in vivo evidence. Cell Metabolism, 27(3), 529–547.
Xiao, W., Wang, R. S., Handy, D. E., & Loscalzo, J. (2018). NAD(H) and NADP(H) redox couples and cellular energy metabolism. Antioxidants & Redox Signaling, 28(3), 251–272.
Yoshino, J., Baur, J. A., & Imai, S. I. (2018). NAD+ Intermediates: The Biology and Therapeutic Potential of NMN and NR. Cell Metabolism, 27(3), 513–528.
Explore
Discover insights on longevity and wellness.
lONGEVITY EXPLAINED
© 2025. All rights reserved.
Terms and conditions
