The Hallmarks of Aging - Why We Age, and What We Can Do About It
7/31/20255 min read

Why Do We Age?
If you’ve ever noticed that some people seem to stay sharp and active well into their 80s while others begin to slow down in their 60s, you’ve seen the mystery of aging play out. The difference isn’t just “good genes” or expensive skincare (though neither hurts). Deep inside, your cells are following a script - one that scientists have finally started to decode.
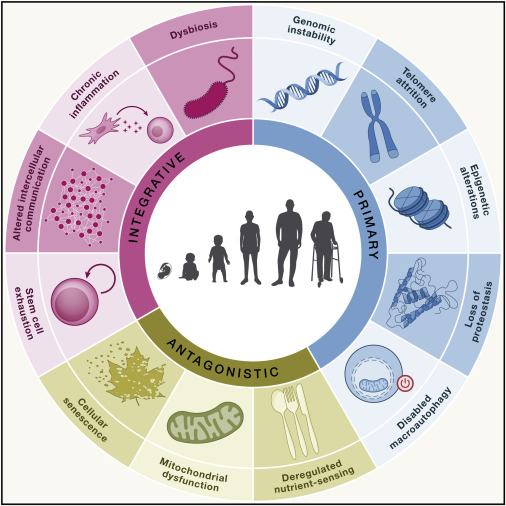
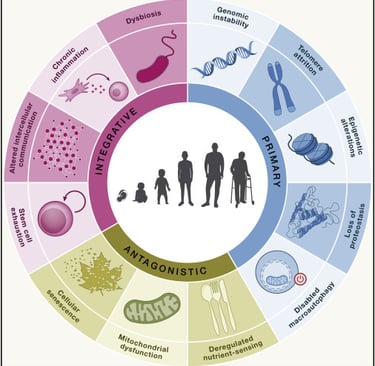
That script is the Hallmarks of Aging: twelve interconnected biological processes that explain much of why we age and how our bodies change. The best part? These hallmarks aren’t just a diagnosis - they’re a roadmap for where we might intervene.
Why This Matters
Once you understand the hallmarks, aging stops feeling like an unstoppable slide and starts looking like a set of levers you can influence. Improve one system, and others often benefit - like tightening a loose screw that stops the whole door from rattling.
From Nine to Twelve: A Quick History
The original framework in 2013 outlined nine hallmarks. A decade of new research expanded this to twelve in 2023, adding three new players: chronic inflammation, microbiome disturbance, and compromised autophagy.
This expansion didn’t just add complexity - it also gave us more entry points for practical action.
The Hallmarks in Three Groups - and How They Connect
Think of the hallmarks like a domino chain:
Primary hallmarks cause the first cracks.
Antagonistic hallmarks are the body’s reactions - helpful at first, harmful if chronic.
Integrative hallmarks are the downstream results that most visibly define aging.
This framework matters because interventions often work upstream - fix a primary cause, and you might prevent a cascade.
Primary Hallmarks - The Root Causes
These are the initial sources of damage that kick-start aging.
Genomic Instability - DNA damage from UV, pollution, and normal metabolism. Your repair systems work constantly but fall behind with age.
Everyday life: Like smudged pages in your instruction manual.
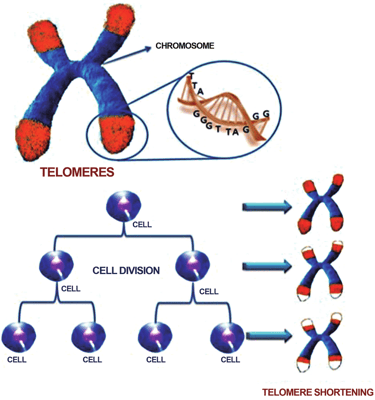
Telomere Attrition - Chromosome endcaps shorten each time cells divide, eventually halting renewal.
Everyday life: A frayed phone charger that finally stops working.
Epigenetic Alterations - Chemical tags on DNA drift, misregulating genes.
Everyday life: You flip the kitchen light, but the bathroom fan turns on.
Loss of Proteostasis - Misfolded or damaged proteins pile up when cleanup systems fail. Linked to Alzheimer’s and Parkinson’s.
Everyday life: Laundry comes out of the dryer in one giant knot.
Disabled Macroautophagy - The cell’s recycling system slows, leading to junk buildup.
Everyday life: The garbage truck comes once a month instead of weekly.
Antagonistic Hallmarks - The Overreactions
These are damage responses that are helpful short-term but harmful long-term.
Deregulated Nutrient Sensing - Pathways like insulin/IGF-1, mTOR, AMPK, and sirtuins get “confused,” promoting growth when repair is needed.
Everyday life: Fuel gauge says “full” when you’re actually on fumes.
Mitochondrial Dysfunction - The cell’s power plants produce less energy and more damaging by-products. Exercise and certain diets can help.
Everyday life: Your phone battery used to last all day… now it dies by lunch.
Cellular Senescence - Damaged cells stop dividing but refuse to leave, secreting inflammatory molecules (the SASP).
Everyday life: The grumpy neighbor who never moves - and throws loud parties.
Integrative Hallmarks - The Endgame
These are the accumulated consequences that directly cause age-related decline.
Stem Cell Exhaustion - Repair slows because stem cells lose function.
Everyday life: Renovation with no builders left on site.
Altered Intercellular Communication - Cell-to-cell messaging falters, often driving chronic inflammation.
Everyday life: The neighborhood WhatsApp group turns into a shouting match.
Chronic Inflammation (“Inflammaging”) - Persistent, low-grade inflammation accelerates tissue breakdown and disease risk.
Everyday life: A quiet fire smoldering in the background.
Microbiome Disturbance - Reduced gut microbial diversity weakens immunity and metabolism.
Everyday life: Your city loses half its workers, and services collapse.
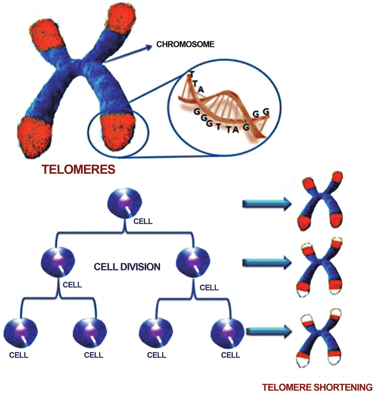
What you see in this image
This graphic shows a chromosome (the packages of DNA inside our cells) with protective caps at the ends called telomeres (highlighted in red). Telomeres are made of repeated DNA sequences - TTAGGG - that act like the plastic tips of shoelaces, preventing the DNA from fraying or sticking together.
Every time a cell divides, telomeres get a little shorter. Over many rounds of cell division, these caps wear down until the cell can no longer divide properly - a process known as telomere shortening. This is one of the classic hallmarks of aging, as it contributes to cell senescence and loss of tissue function.
Why this matters for longevity
Telomere shortening isn’t just about “running out of cell divisions.” It also connects with other hallmarks, like DNA damage and mitochondrial dysfunction. Later in this series, when we discuss NAD+ and related supplements (like NR and NMN), this picture will come back into focus - because NAD+ is deeply involved in DNA repair and cellular stress responses, processes that may influence how telomeres function and age.
Everyday Relevance (Quick Wins You Can Actually Feel)
A brisk 30-minute walk hits multiple hallmarks at once: improves mitochondrial function, nutrient sensing, and lowers inflammation.
Plant- and fiber-rich meals nourish your microbiome and calm chronic inflammation (think beans, veggies, nuts, olive oil).
Consistent sleep repairs DNA, balances hormones, and supports brain health (Why We Sleep - Walker, 2017).
Stress buffers like breath work and social connection lower harmful inflammation.
Many habits hit multiple hallmarks at once.
Myth Check: The Overnight Hack
The internet loves the promise of “reverse aging in 30 days.” Biology doesn’t.
But not everything is hype either. Some tools - omega-3s, vitamin D, creatine in older adults, and yes, certain NAD+ boosters - have promising evidence for supporting healthy aging when used appropriately (we’ll dig into NAD+ soon). The key is understanding mechanism, dose, quality, and fit before buying.
I’m not anti-supplement. I'm anti-wasting your money.
Emerging Frontiers - What’s Around the Corner?
Beyond habits, science is steering toward hallmark-specific tools:
Autophagy boosters: compounds and protocols being tested to nudge cellular recycling in older adults.
Microbiome restoration: targeted probiotics/prebiotics to rebuild diversity and reduce inflammation as we age. (Microbiome is the collection of all microbes (like bacteria, fungi, and viruses) and their genes that naturally live on and in the human body.)
Gene-expression tuning: small molecules and (eventually) gene therapies to activate DNA repair or quiet pro-inflammatory signals.
Wearable-driven programs: sleep, glucose, and activity data feeding AI-coached routines to hit nutrient sensing, inflammation, and mitochondrial health together.
Biological age dashboards: future “biological age profiles” that show which hallmarks are most off-track - and how to correct them.
The Road Ahead
Later in this blog: we’ll break down each hallmark in its own post - what it is, how to measure it (if possible), and what the best current evidence says you can do. We’ll also review supplements and interventions with clear context (who might benefit, who shouldn’t, what dose/form, and what’s still unknown).
If you stick around, you’ll learn why terms like telomeres, mitochondria, autophagy, and microbiome keep popping up - and you’ll know how to make them work for you.
Light “Further Reading”
For those interested and impatient for my in-depth Hallmarks review, you can take a peak into following articles. These are the basics to understand how this actually works.
“Hallmarks of Stem Cell Aging” - Rando et al., Cell Stem Cell, 2025
References
López-Otín, C., Blasco, M. A., Partridge, L., et al. (2013). The hallmarks of aging. Cell, 153(6), 1194–1217.
López-Otín, C., Kroemer, G., Partridge, L., et al. (2023). Hallmarks of aging: An expanding universe. Cell, 186(2), 243–278.
Rajman, L., Chwalek, K., & Sinclair, D. A. (2018). Therapeutic potential of NAD-boosting molecules: The in vivo evidence. Cell Metabolism, 27(3), 529–547.
Walker, M. (2017). Why we sleep: Unlocking the power of sleep and dreams. Scribner.
Explore
Discover insights on longevity and wellness.
lONGEVITY EXPLAINED
© 2025. All rights reserved.
Terms and conditions
